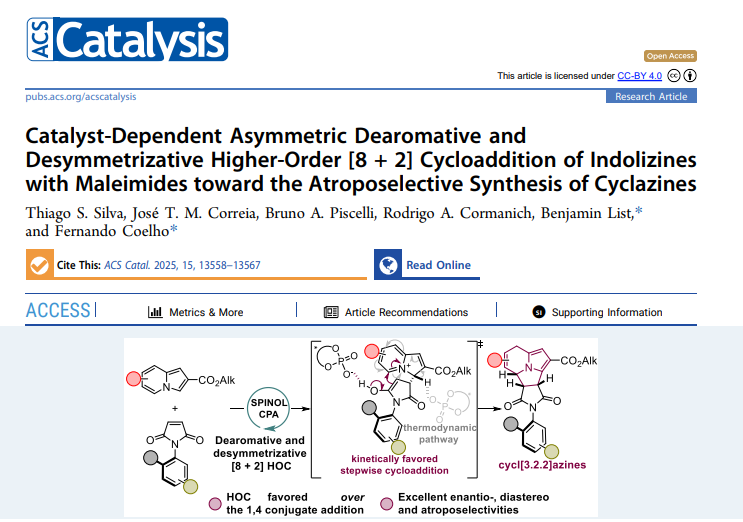
We are pleased to share that our new open‑access article, “Catalyst‑Dependent Asymmetric Dearomative and Desymmetrizing Higher‑Order [8 + 2] Cycloaddition of Indolizines with Maleimides toward the Atroposelective Synthesis of Cyclazines,” has just been published in ACS Catalysis (22 July 2025). The study is the culmination of Thiago S. Silva’s experimental work—carried out as part of his PhD stay in Prof. Benjamin List’s laboratory—and Bruno A. Piscelli’s in‑depth theoretical analysis performed in our group. Thiago discovered that finely tuned chiral phosphoric acids redirect the reaction away from classical Friedel–Crafts addition and into a dearomative higher‑order [8 + 2] cycloaddition, providing atropisomeric cyclazines with enantioselectivities up to 98:2 e.r. Bruno’s DFT calculations then revealed a kinetically favored stepwise pathway and clarified how the catalyst acts as a proton shuttle in the key isomerization step, explaining both the chemoselectivity and the remarkable stereocontrol observed in the lab. This synergy between Thiago’s synthetic insight and Bruno’s computational rigor exemplifies the collaborative spirit of our group and opens new avenues for the concise construction of atropisomeric N‑heterocycles. The paper is freely available under a CC‑BY 4.0 license—feel free to download it at: https://doi.org/10.1021/acscatal.5c03020
Related Posts
Chemistry World article
01/15/2026
New paper in Angewandte Chemie
11/11/2025