A recent article in Chemistry World revisits one of the most discussed stereoelectronic phenomena in organic chemistry: the anomeric effect. Instead of reinforcing the traditional, single-cause explanation based on hyperconjugation, the piece reports new computational evidence showing that the effect emerges from a balance of multiple contributors. Based on a detailed study led by Igor…
Author: cormanich
New paper in Angewandte Chemie: Improper hydrogen bonding in RCH₂F and RCHF₂ motifs Congratulations to Bruno A. Piscelli for his new paper published in Angewandte Chemie! This paper was done in collaboration with David O’Hagan and Michael Bühl from the University of St Andrews. In this work, we use high-level quantum-chemical calculations to understand how…
Our new article, “Performance of Semiempirical DFT Methods for the Supramolecular Assembly of Janus-Face Cyclohexanes,” is out in Physical Chemistry Chemical Physics. We benchmark GFN-xTB against high-level DFT/ab initio for conformational equilibria and supramolecular stacking, and show that DFT single-point corrections on GFN-optimized geometries reach near-DFT-D3 accuracy at much lower cost. This enables faster, reliable…
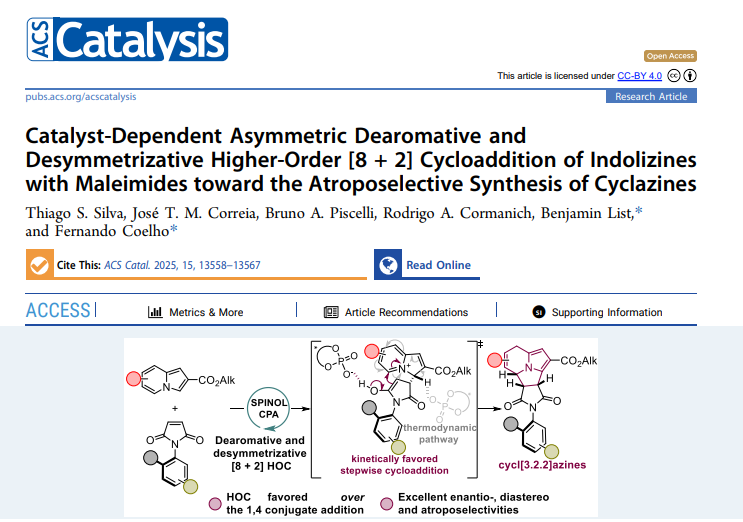
We are pleased to share that our new open‑access article, “Catalyst‑Dependent Asymmetric Dearomative and Desymmetrizing Higher‑Order [8 + 2] Cycloaddition of Indolizines with Maleimides toward the Atroposelective Synthesis of Cyclazines,” has just been published in ACS Catalysis (22 July 2025). The study is the culmination of Thiago S. Silva’s experimental work—carried out as part of his PhD stay in Prof. Benjamin List’s laboratory—and Bruno A. Piscelli’s in‑depth…
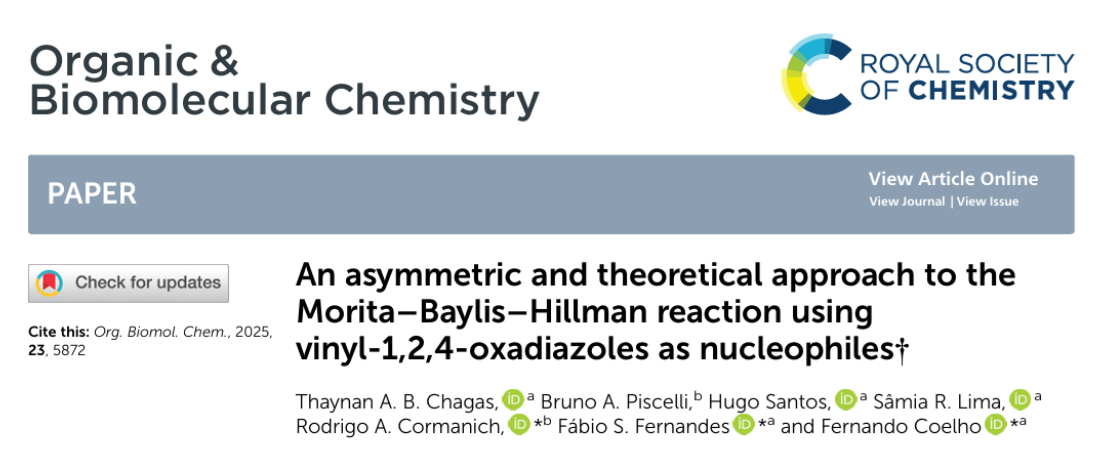
We are pleased to share our latest collaborative work with Professor Fernando Coelho’s group, now published in Organic & Biomolecular Chemistry: “An asymmetric and theoretical approach to the Morita–Baylis–Hillman reaction using vinyl-1,2,4-oxadiazoles as nucleophiles”† Thaynan A. B. Chagas, Bruno A. Piscelli, Hugo Santos, Sâmia R. Lima, Rodrigo A. Cormanich,* Fábio S. Fernandes,* and Fernando Coelho*https://doi.org/10.1039/d5ob00540j…
Excited to share our latest collaborative work with the Jurberg group: “Visible Light-Mediated Preparation of a Key Intermediate Employed in the Synthesis of Zolpidem and Several Analogs” In this study, we developed a visible light-driven C3-alkylation protocol for imidazo[1,2-a]pyrimidines and imidazo[1,2-a]pyridines using aryldiazoacetates. This strategy provides efficient access to known and novel analogs of a…
In our latest publication, we explore the synthesis and reactivity of six-membered hypervalent bromine(III) and chlorine(III) compounds, broadening the scope of halogen chemistry beyond iodine analogs. This study is the result of a collaboration with Professor Thomas Wirth’s lab, where we combined synthetic efforts with computational investigations to elucidate key mechanistic aspects. We introduce novel…
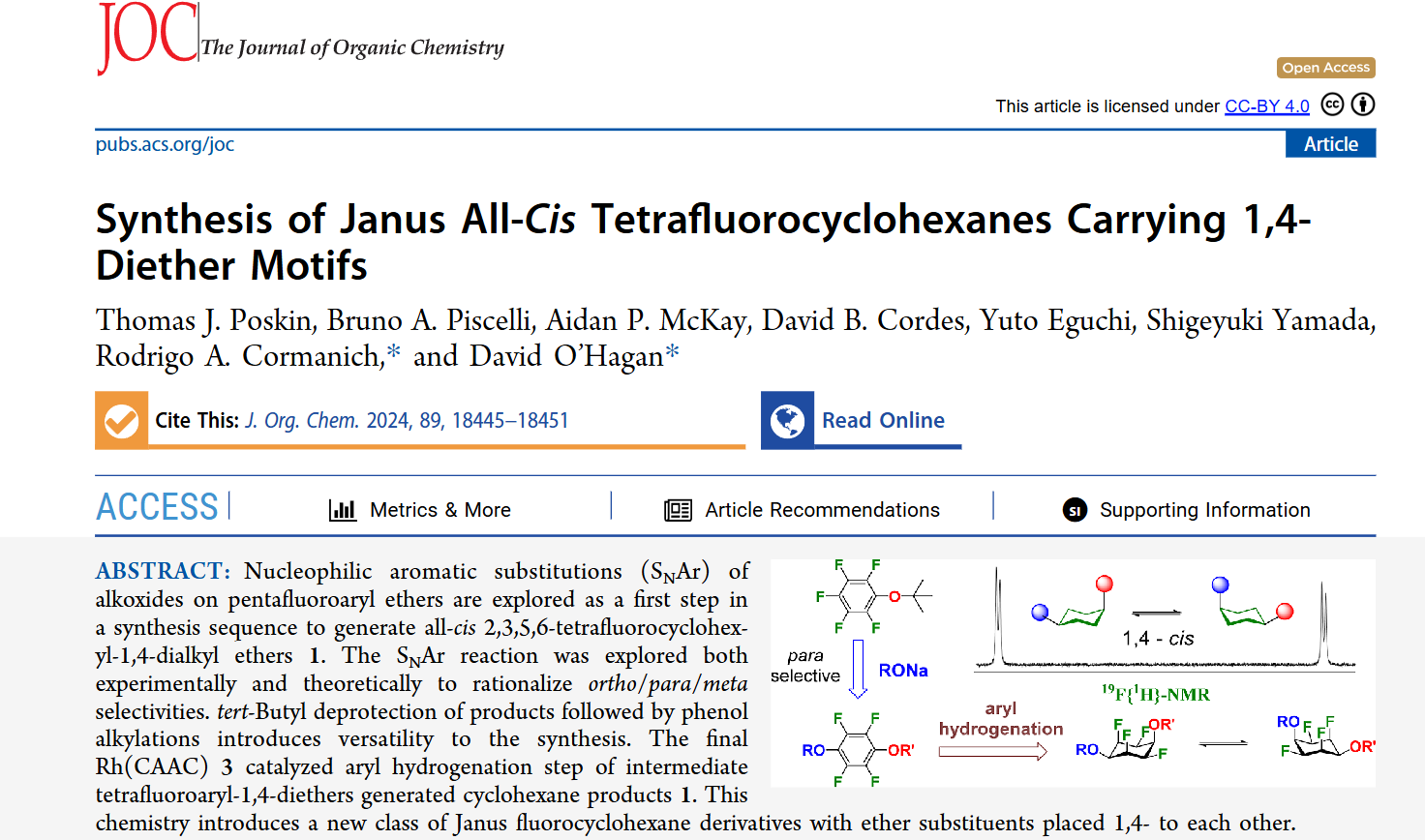
We are pleased to announce our new publication, in collaboration with Professor David O’Hagan’s group, showcasing a combined experimental and computational study. Our research focused on synthesizing a new class of “Janus” fluorocyclohexanes featuring 1,4-diether substituents. We first investigated nucleophilic aromatic substitutions (SNAr) of alkoxides on pentafluoroaryl ethers, using both bench work and theoretical calculations…
We are thrilled to announce the publication of our latest research paper in Chemical Communications, the result of a fruitful collaboration with Professor Igor Jurberg. The study presents a groundbreaking reaction sequence that combines visible light-mediated cyclopropanation with acid-promoted ring-opening, leading to the formal alkylation of silyl enol ethers with aryldiazoacetates. In this work, we…
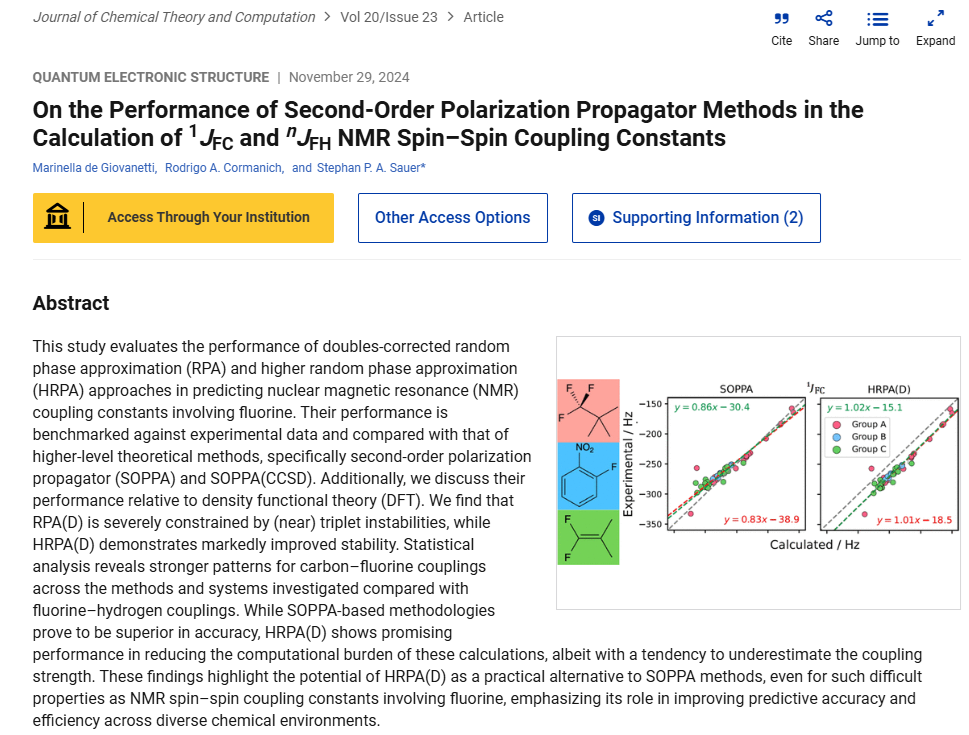
In this work, we evaluate the performance of doubles-corrected random phase approximation (RPA) and higher random phase approximation (HRPA) methods in predicting NMR spin–spin coupling constants involving fluorine. By benchmarking these approaches against experimental data and comparing them to SOPPA, SOPPA(CCSD), and density functional theory (DFT), we demonstrate that while RPA(D) is hindered by (near)…