We are excited to announce the publication of our latest research in the Journal of Organic Chemistry (JOC), where we delve into the conformational equilibria of selectively halogenated cyclohexanes using a combination of experimental and computational methods.
In our study, we employed Variable Temperature Nuclear Magnetic Resonance (VT-NMR) experiments to investigate 1,1,4-trifluorocyclohexane 7 and conducted computational analyses at the M06-2X/aug-cc-pVTZ level to explore a wider range of highly fluorinated cyclohexanes.
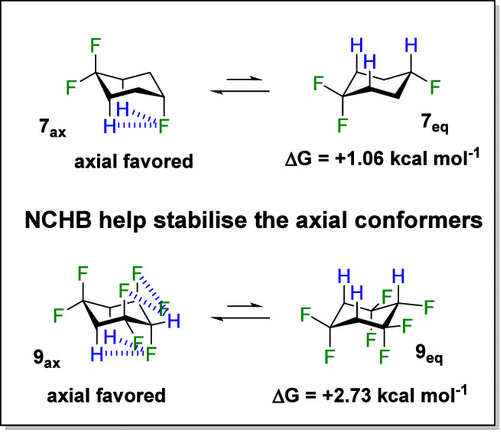
Surprisingly, our findings revealed an unexpected preference for the axial conformation (7ax) over the equatorial conformation (7eq) in 1,1,4-trifluorocyclohexane 7, with a ΔG of 1.06 kcal mol–1, contrary to the conventional understanding of substituent effects on cyclohexanes. This axial preference was even more pronounced in 1,1,3,3,4,5,5-heptafluorocyclohexane 9, with a ΔG of 2.73 kcal mol–1, attributed to the increased polarization of isolated CH2 hydrogens by CF2 groups.
Further analysis using natural bond orbital (NBO) methods revealed the significant contribution of nonclassical hydrogen bonding (NCHB) between the C-4 fluorine and the diaxial hydrogens at C-2 and C-6 in cyclohexanes 7 and 9, accounting for the observed conformational bias.
Additionally, our study extended to halogen substitutions (Cl, Br) at the pseudoanomeric position, uncovering their influence on conformer stabilities through electrostatic interactions and geminal −CHX– hydrogen polarization.
These findings provide valuable insights into the conformational preferences of halogenated cyclohexanes and highlight the role of nonclassical hydrogen bonding in modulating their structural dynamics. We look forward to further exploring these intriguing phenomena in future research endeavors.
Stay tuned for more exciting updates from our research group!