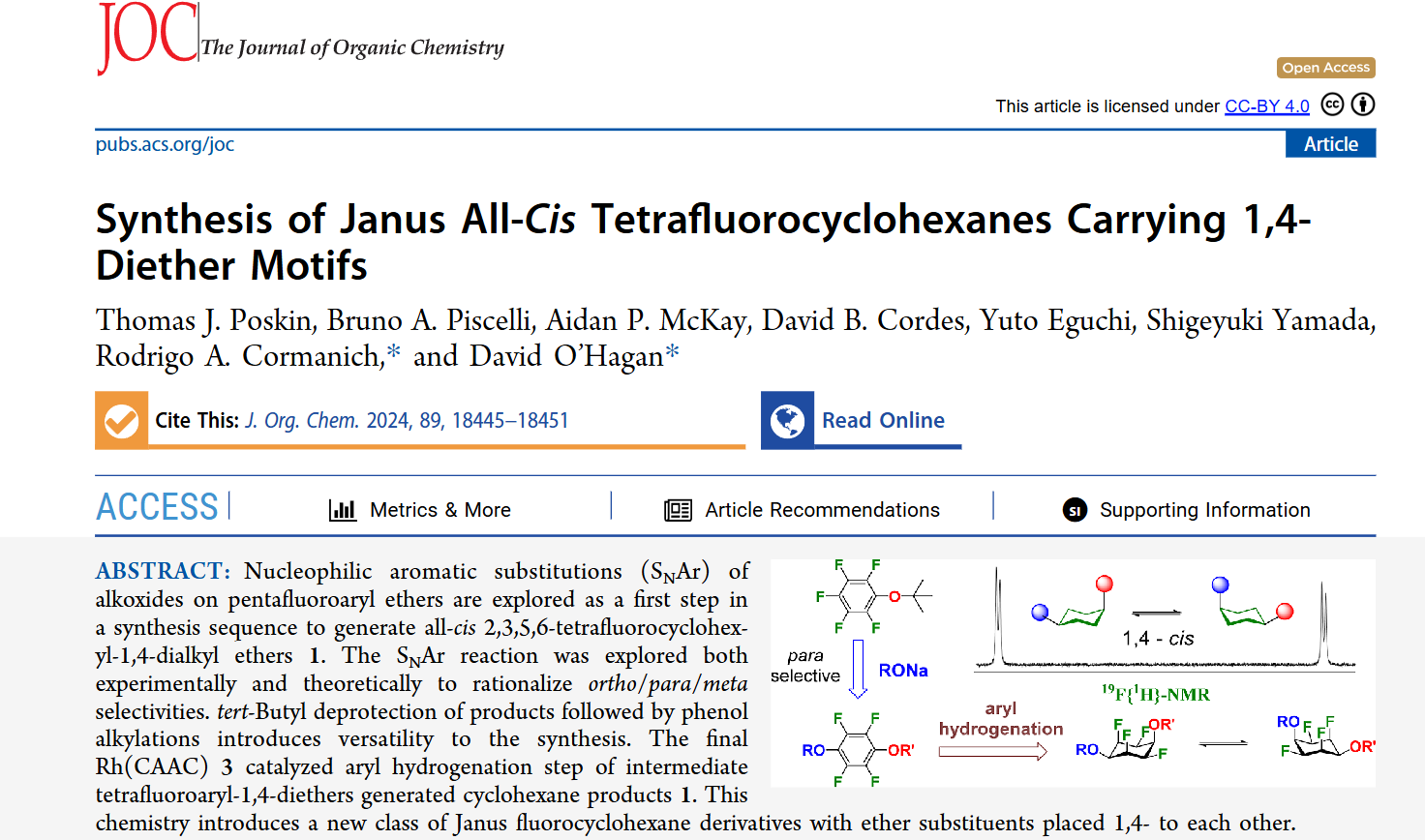
We are pleased to announce our new publication, in collaboration with Professor David O’Hagan’s group, showcasing a combined experimental and computational study. Our research focused on synthesizing a new class of “Janus” fluorocyclohexanes featuring 1,4-diether substituents. We first investigated nucleophilic aromatic substitutions (SNAr) of alkoxides on pentafluoroaryl ethers, using both bench work and theoretical calculations to understand regioselectivity. Subsequent deprotection and alkylation steps allowed for structural diversification, and a final hydrogenation using Rh(CAAC)3_33 led to the all-cis tetrafluorocyclohexane targets. These versatile molecules pave the way for innovative applications in materials science and beyond. Check out our paper for more details!
Related Posts
Chemistry World article
01/15/2026
New paper in Angewandte Chemie
11/11/2025