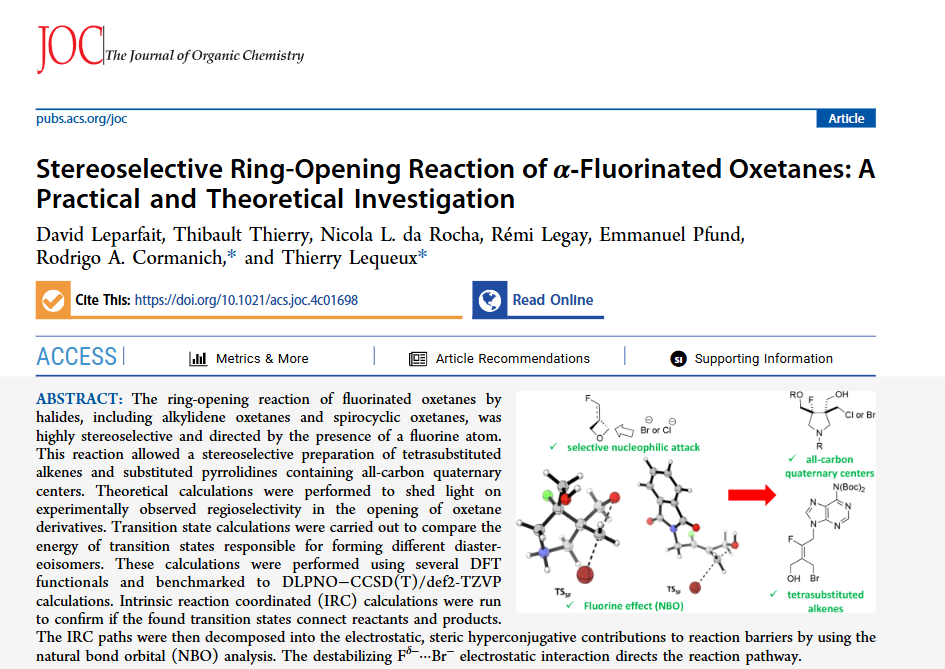
We’re excited to present our latest findings on the ring-opening reaction of fluorinated oxetanes by halides. This highly stereoselective reaction, directed by the presence of a fluorine atom, enabled the preparation of tetrasubstituted alkenes and pyrrolidines with all-carbon quaternary centers—valuable compounds in synthetic chemistry.
On the computational side, transition state calculations illuminated the regioselectivity of the reaction. These studies, benchmarked with high-level methods. Using NBO analysis, we uncovered that a destabilizing Fδ−···Br⁻ electrostatic interaction is a key factor shaping the reaction outcome.
This work underscores the synergy between experimental and theoretical approaches in advancing modern organic synthesis. Thanks a lot the Lequeux lab for all the collaborative work;