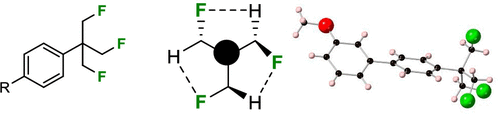
We’re excited to share our latest research spotlight on the (β,β′,β″-trifluoro)-tert-butyl (TFTB) group, a molecule that has historically been overlooked in chemical literature. We owe a significant part of this research endeavor to our collaboration with the renowned David O’Hagan Lab. Working alongside such an esteemed team allowed for an amalgamation of expertise and innovative perspectives.
Our work offers a fresh perspective by showcasing a direct synthesis of the TFTB group. One of the intriguing features of the TFTB group is its derivation – it’s what you get when the methyl groups of a tert-butyl moiety are replaced by fluoromethyl groups. This seemingly small modification has a profound impact on its characteristics. Sequential fluoromethylations lead to a noticeable decrease in Log P, signifying an increase in hydrophilicity.
But our exploration doesn’t stop there. Delving deeper into the TFTB group, our research emphasizes its synthetic transformations, its conformational intricacies, and its metabolism. All these findings indicate that the TFTB group possesses a promising profile that could potentially be pivotal in the realm of bioactive compound discovery.
Check it at Organic Letters: https://pubs.acs.org/doi/10.1021/acs.orglett.3c02236